|

November 15, 2023
By
Gwangju Institute of Science and Technology
Researchers improve water splitting
reaction for green hydrogen gas production
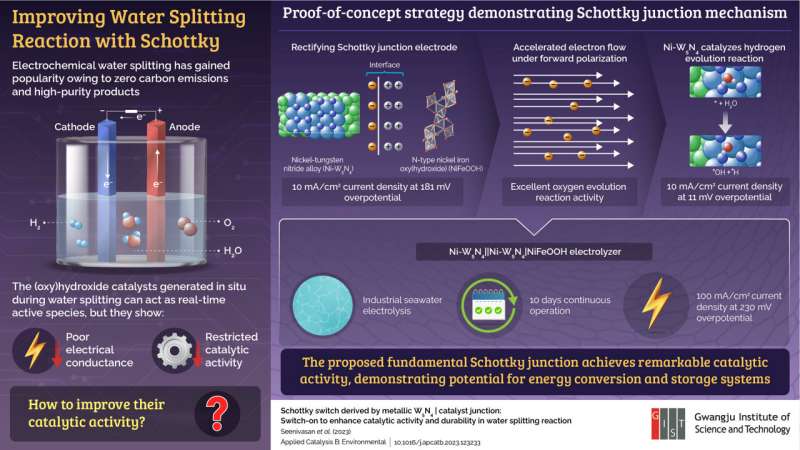
Scientists demonstrate a Schottky junction with
metallic Ni-W5N4 and n-type NiFeOOH interface to overcome the
conductance limit of (oxy)hydroxide species, producing hydrogen gas
via water splitting reactions. Credit: Junhyeok Seo from GIST
Green hydrogen (or H2) produced from renewable
energy resources is the fuel of a decarbonized future. Electrolysis,
or splitting of water into oxygen and hydrogen with the help of an
electrochemical cell, is one of the most popular ways of producing
green H2.
It is a simple reaction, ensures high-quality products, and has zero
carbon emissions. Despite its advantages, however, electrochemical
water splitting has yet to gain prominence on a commercial scale. This
is because of the low electrical conductivity of active (oxy)hydroxide
catalysts generated in situ during the electrochemical processes.
This, in turn, leads to restricted catalytic activity, hampering
hydrogen as well as oxygen evolution reactions in the cell.
The problem of (oxy)hydroxide's poor electrical properties has been a
long-standing challenge to the achievement of efficient water
splitting. Now, a team of researchers led by Associate Professor
Junhyeok Seo from the Department of Chemistry at Gwangju Institute of
Science and Technology, has found a solution to this issue in the form
of Schottky junctions.
In a recent study published in Applied Catalysis B: Environmental,
they demonstrated an electrode with a Schottky junction formed at the
interface of metallic nickel-tungsten nitride (Ni-W5N4) and
semiconducting n-type nickel-iron (oxy)hydroxide (NiFeOOH) catalyst.
This electrode was able to overcome the conductance limit of (oxy)hydroxide
and improved the water-splitting ability of the setup.
Notably, two materials, a metal, and a semiconductor, with largely
different electronic behaviors, were put in contact to make an energy
difference at the interface, forming a junction. "Our research
utilized this potential energy barrier present in the Schottky
junction to accelerate electron flow in the electrode, leading to a
significant increase in oxygen evolution reaction activity, expediting
overall water splitting," explains Dr. Seo, highlighting the core
mechanism behind their newly designed electrode.
Upon carrying out electrocatalytic water splitting, the team observed
that Ni-W5N4 alloy catalyzed the hydrogen evolution reaction,
resulting in 10 mA/cm2 current density at a small overpotential of 11
mV. Furthermore, the rectifying Schottky junction formed at the
interface of Ni-W5N4|NiFeOOH nullified the non-conductive lamination
produced by (oxy)hydroxide species.
In forward bias, it exhibited a current density of 11 mA/cm2 at 181 mV
overpotential. The electrochemical analysis of the electrode revealed
that the improved catalytic activity could indeed be attributed to the
Schottky junction.
Lastly, the researchers designed an electrolyzer using their Schottky
junction electrode for industrial seawater electrolysis. They found
that the new device could operate continuously for 10 days, while also
exhibiting outstanding catalytic activity and durability during
electrolysis. It showed a remarkable current density of 100 mA/cm2 at
an overpotential of just 230 mV.
Overall, the researchers believe that these findings can contribute
toward a sustainable strategy for hydrogen production to eventually
replace conventional methods that still rely on fossil fuels. As Dr.
Seo concludes, "Freshwater and seawater are abundant and renewable
sources of protons. Efficient water splitting systems ensure that we
can establish sustainable production of zero carbon hydrogen fuel,
thus helping manage our current climate problems."
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|