|

By
Yasmin
Ahmed Salem, Max
Planck Society
31 Aug, 2023
Examining challenges in green hydrogen production:
Study finds hydrogen effects that produce bottleneck
Graphical abstract.
Credit: ACS Energy Letters (2023). DOI: 10.1021/acsenergylett.3c00842
The quest for hydrogen as a clean and sustainable energy source has
gained momentum. To produce green hydrogen, water must be split into
oxygen and hydrogen. This water splitting process is facilitated by
electrocatalysts that enhance the chemical reaction rate.
Ideally, a catalyst is
neither changed nor degraded by the reaction, and for electrolyzers
this becomes critical as the electrocatalysts account for 50% of its
total cost. As a result, their efficiency and lifetime are critical to
the future availability of green hydrogen and
thus to a carbon-free economy. A team of researchers led by the
Max-Planck-Institut für Eisenforschung (MPIE) has discovered why these
catalysts actually deteriorate and suffer from a shorter life
expectancy.
Their work shows that the produced hydrogen itself is the bottleneck.
The scientists have now published their findings in the journal ACS
Energy Letters.
Effects of hydrogen on catalytic performance
Previous research
primarily focused on optimizing catalyst performance, without
atomic-level analysis. However, the Max Planck team took a different
approach.
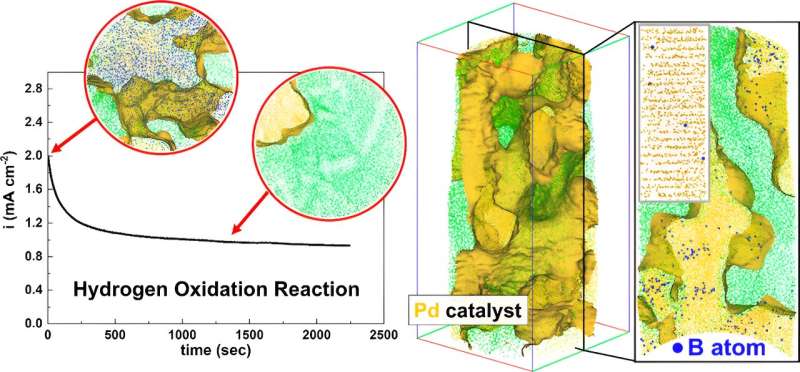
"Our findings revealed that impurities do get introduced during
synthesis. Surprisingly, we discovered that boron impurities could
enhance the catalyst's performance by expanding its lattice structure.
However, we observed that the catalytic
activity decreases after a certain amount of hydrogen is produced
and wanted to understand why this happens to find ways to maintain the
performance," explains Prof. Baptiste Gault, corresponding author of
the publication and head of the group "Atom Probe Tomography" at MPIE.
Atom probe tomography and simulations based on density functional
theory revealed that as hydrogen accumulates on the catalyst's
surface, boron is gradually removed from the lattice structure. This
interaction deteriorates the catalyst's performance, by decreasing the
concentration of boron dopants.
"Our findings show that it is not enough to increase the catalytic
activity with Boron as a dopant. We must find solutions to shield
Boron inside the catalyst's lattice
structure from the hydrogen produced on the surface of the
catalyst," says Prof. Se-Ho Kim, second corresponding author of the
publication, former postdoctoral researcher at MPIE and now assistant
professor at Korea University.
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|