|

September 6, 2023
By Tsinghua University Press
New catalyst decreases the energy
required to split hydrogen gas from water
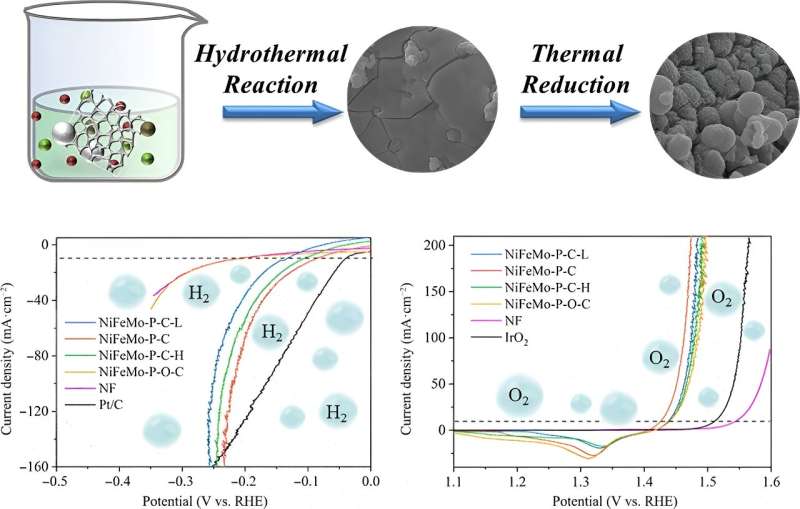
Upper diagram: The NiFeMo-P-C catalyst is
synthesized by mixing an aqueous solution of metal salts and sodium
hypophosphite, a sodium salt of a phosphorus-containing acid, with
treated nickel foam and subjecting the solution to a simple, low-cost
hydrothermal reaction that increases the temperature and pressure of
the solution in the reaction vessel. The intermediate product [middle
scanning electron microscope (SEM) image] is then loaded with alloy
and metal phosphide through H2/Ar (hydrogen/argon) thermal reduction
(adding electrons to the metal ions using hydrogen and heat) to create
the final catalyst product (right SEM image). Lower graphs: Graphs
depicting the linear sweep voltammetry, or current density of the
working electrode in the hydrogen evolution reaction (left graph) and
oxygen evolution reaction (right graph) at different potentials,
depending on the catalyst used. NiFeMo-P-C performance is labeled in
red. Credit: Nano Research Energy, Tsinghua University Press
Hydrogen gas is a clean, renewable alternative to
fossil fuels, but current industrial production methods used to
produce hydrogen release carbon into the atmosphere and pollute the
environment.
A new catalyst, carbon compound nickel-iron-molybdenum-phosphide
anchored on nickel foam (NiFeMo-P-C), has significantly decreased the
amount of electricity required to generate both hydrogen and oxygen
from water, providing a clean and efficient means to produce hydrogen
gas.
A team of leading chemical engineers have synthesized a
cost-efficient and easily manufactured catalyst designed to lower the
amount of energy required for the electrolysis of water, which splits
water molecules into hydrogen and oxygen using electricity.
Hydrogen and oxygen gas are split from water through the
hydrogen evolution reaction (HER) and oxygen evolution reaction (OER),
respectively. The transition metal alloy, or mixture containing at
least one metal, nickel-iron-molybdenum (NiFeMo) was used as a
catalyst for water electrolysis due to the incomplete filling of
electron orbitals in transition metal atoms nickel and iron, making it
an ideal electron donor and acceptor in chemical reactions. Phosphide
was added to the catalyst to improve corrosion resistance in an
alkaline, or basic pH, electrolyte solution.
The team published the results of their study in Nano
Research Energy on July 7.
"Hydrogen is recognized as the most ideal alternative to
fossil fuels due to its high… energy density, high heat conversion
efficiency and zero carbon emission. However, commonly applied
hydrogen production methods in industry, including steam reforming of
natural gas and methanol and gasification of coal, consume fossil
fuels and cause serious pollution to the environment," said Jingjing
Tang, supervisor of the study and associate professor at Central South
University in Changsha, China.
"Water electrolysis takes water as raw material to
produce high-purity hydrogen by converting electricity into chemical
energy, which is a clean and promising hydrogen production
technology," said Tang.
Catalysts used to lower the energy required for both the
HER and OER existed previously, but utilized platinum and iridium
oxide, valuable elements that are both expensive and in short supply.
Creating an affordable catalyst that lowers the activation energy of
both reactions reduces overall manufacturing costs and improves the
commercial viability of clean hydrogen gas production.
One challenge in designing a bifunctional catalyst was
the special requirements of the OER. "Because OER is a four-electron
transfer reaction with sluggish kinetics, [it] generally performs
better in alkaline solution. It was critical to research non-noble,
metal-based electrocatalysts with excellent bifunctional performance
in [an] alkaline electrolyte," said Tang. The team created the alloy
and metal phosphide to maintain catalyst integrity in these alkaline
conditions.
To test the composition and valence state of the
generated NiFeMo-P-C catalyst, the team subjected the compound to
X-ray photoelectron spectroscopy (XPS) measurement to confirm the
presence of Ni, Fe, Mo, P, C and O. The high-resolution spectrum of
nickel also identified 2p3/2 and 2p1/2 spin orbits, which refers to
the state of electrons in the nickel atoms of the catalyst.
Overall, the newly developed NiFeMo-P-C electrocatalyst
requires very low overpotentials, or energy required to split water,
for HER (87 mV to achieve a current density of 10 mA·cm–2) and OER
(196 mV to achieve a current density of 10 mA·cm–2). The cell voltage,
or difference in voltage between two electrodes, required for water
electrolysis using the catalyst is also only 1.50 V at 10 mA·cm–2.
The team is optimistic that their discovery will make
clean hydrogen production a reality. "Unlike most bifunctional
catalysts, NiFeMo-P-C can achieve excellent catalytic performance
without complicated preparation steps and elaborate nanostructures.
Besides, the superior durability without any [voltage] attenuation
within 50 hours... makes NiFeMo-P-C an ideal [non-precious metal
catalyst] candidate… for large-scale hydrogen production," said Tang.
Other contributors include Xiangyang Zhou, Tingting
Yang, Ting Li, Youju Zi, Sijing Zhang, Lei Yang, Yingkang Liu and Juan
Yang from the School of Metallurgy and Environment at Central South
University in Changsha, China.
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|