|

January 11, 2024
Argonne researchers use AI to
downselect candidates
for liquid organic hydrogen carriers from 160 billion to 41
In a computational study leveraging artificial
intelligence (AI), scientists at the US Department of Energy’s (DOE)
Argonne National Laboratory assessed 160
billion molecules to screen the molecules for suitability as liquid
carriers of hydrogen.
The team whittled down the candidates to a mere
41; the task passes into the hands of experimentalists now to test the
promising ones. An open-access paper on the work is published in the
RSC journal Digital
Discovery.
We
present a comprehensive, in silico-based discovery approach to
identifying novel liquid organic hydrogen carrier (LOHC) candidates
using cheminformatics methods and quantum chemical calculations. We
screened over 160 billion molecules from ZINC15 and GDB-17 chemical
databases for structural similarity to known LOHCs and employed a
data-driven selection criterion connecting molecular features with
dehydrogenation enthalpy. This scoring criterion effectively
predicts dehydrogenation enthalpies from SMILES strings,
streamlining the LOHC screening process.
After rigorous screening and
down-selection, we compiled a database of 3000 dehydrogenation
reactions for the most promising LOHC candidates, setting the stage
for future selection based on kinetics and catalysis. This work
demonstrates the significant impact of integrating quantum chemistry
and cheminformatics in materials discovery, accelerating the
selection process while reducing experimental efforts and time.
By
proposing new molecules as prospective LOHC candidates, our study
provides a valuable resource for researchers and engineers in the
development of advanced LOHC systems and showcases a successful
approach for high-throughput discovery, contributing to more
efficient and sustainable energy storage solutions.
—Harb et
al.
Hydrogen in its pure form exists as a gas under
normal conditions. For use as a fuel, one of the challenges is
shipping this gas safely to refueling stations and storing it.
Hydrogen carrier compounds in liquid form offer several advantages.
They have a much better safety profile because they are not as prone
to leaking and explosion. They also have a much higher energy content
per unit volume, making storage and transportation far easier.
The most visible form of a liquid hydrogen
carrier compound is water—two atoms of hydrogen and one of oxygen.
Another form is organic molecules, essentially an endless number of
possible combinations of hydrogen and carbon atoms, in addition to
other atoms such as nitrogen and oxygen.
Among the billions of possible liquid hydrogen
carriers, common examples include chemicals like ammonia and methanol.
However, the relatively few candidates tested in the laboratory to
date have suffered from chemical instability and unwanted side
reactions.
The team screened the candidate molecules based
on four factors.
-
Structural similarity to known liquid
hydrogen carriers.
-
Desirable physical properties, such as melting
and boiling points—the liquid must stay liquid when the hydrogen has
been added or extracted.
-
Storage capacity.
-
Dehydrogenation enthalpy.
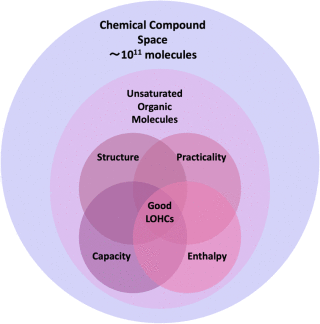
Visual representation of the search space and approach
for identifying suitable LOHCs. The large outer circle represents the
entire chemical compound space, estimated to contain around 1011 molecules.
The smaller circle within this represents the subset of these
molecules that are unsaturated. Within this subset, a four-circle Venn
diagram represents additional selection criteria, labeled as
Structure, Practicality, Capacity, and Enthalpy, and they correspond
to structural similarity to known LOHCs, desirable physical properties
and molecule synthesizability, a gravimetric capacity of 5.5% or
higher, and a dehydrogenation enthalpy in the 40–70 kJ per mol H2 range,
respectively. The researchers hypothesize that the intersection of
these four criteria indicates the most promising candidates for LOHCs.
Harb et
al.
The team’s calculations necessitated access to
supercomputers available at few places in the world. One of them is
Argonne, home to the Argonne Leadership Computing Facility, a DOE
Office of Science user facility. The team also relied on Bebop, a
computing cluster operated by the Laboratory Computing Resource Center
at Argonne.
Even with these powerful resources available, if
one allots one millisecond of compute time per molecule, that
translates into five years of compute time for 160 billion molecules.
For that reason, the team developed an AI-based screening approach
that sped up the computations to three million molecules per second,
or about 14 hours for the 160 billion.
Resources
-
Hassan Harb, Sarah N. Elliott, Logan
Ward, Ian T. Foster, Stephen J. Klippenstein, Larry A. Curtiss and
Rajeev Surendran Assary (2023) “Uncovering novel liquid organic
hydrogen carriers: a systematic exploration of chemical compound
space using cheminformatics and quantum chemical methods” Digital
Discovery doi: 10.1039/D3DD00123G
Green Play Ammonia™, Yielder® NFuel Energy.
Spokane, Washington. 99212
509 995 1879
Cell, Pacific Time Zone.
General office:
509-254
6854
4501 East Trent
Ave.
Spokane, WA 99212
|